Abstract:
Direct detonation initiation by a point energy source in a rich H2–NO2/N2O4–Ar mix- ture has been investigated using high-resolution one-dimensional numerical simulations performed with detailed chemistry. In particular, the effect of the 2-step energy release profile on the critical initiation energy and the dynamics of the failure process has been studied for the first time. The critical initiation energy for successful detonation initiation decreases as the second step of heat release is strengthened and the width of the overall heat release pulse is reduced. This feature has been investigated by studying the competition between chemical heat release and unsteadiness along the path of Lagrangian particles. The decrease of the critical initiation energy for stronger second step of heat release seems to be a result of the decrease of the sensitivity of this second step to unsteadiness-induced quenching behind a decaying shock wave.
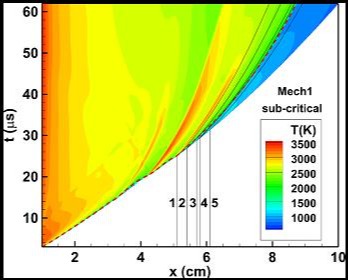
x-t diagram and temperature field of a sub-critical detonation initiation
in a rich H2-NO2/N2O4 mixture
[1] Faghih M., Mével R., He. Y., and Chen Z.:
Effect of 2-step energy release on the direct detonation initiation in a rich H2-NO2/N2O4-Ar mixture. Combustion and Flame, 2020, vol 222, p 317-325.
[2] He Y., Liu Y.C., and Mével R.: Effect of volumetric expansion on shock-induced ignition of H2-NO2/N2O4 mixtures
Combustion and Flame, 2020, vol 215, p 425-436


