Abstract:
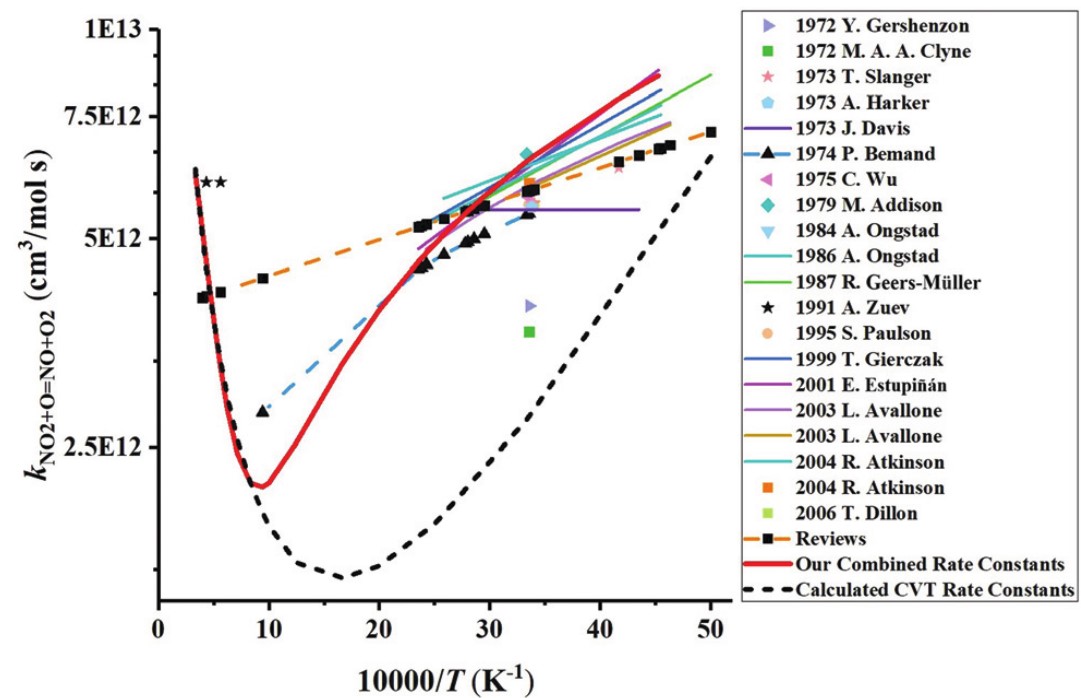
A chemically consistent rate constant for the reaction between nitrogen dioxide and the oxygen atom has been obtained by combining low-temperature experimental data from the literature and new high-temperature quantum chemical calculations. The effect of the inclusion of the new rate constant on the prediction of three detailed reaction models from the literature has been studied using (i) new experimental oxygen atom profiles obtained in a shock tube during nitrogen dioxide pyrolysis, and (ii) published shock tube and jet-stirred reactor data for H2–NOx mixtures with and without dioxygen. Overall, the predictive capability of the reaction models were improved. The present study suggests that our chemically consistent rate constant should be included in detailed reaction models for combustion applications.
Arrhenius plot for the reaction NO2+O=NO+O2
Li Y., Javoy S., Mével R., and Xu X.: A chemically consistent rate constant for the reaction of nitrogen dioxide with oxygen atom.
Physical Chemistry Chemical Physics, 2021, vol 23, p 585-596


