Research progress on the reaction mechanism of trisulfur radical generation in Li-S batteries by Xuefei Xu’s group at Center for Combustion Energy and Department of Energy and Power Engineering of Tsinghua University
Recently, a paper was published in Journal of Materials Chemistry A by Xu Han and others from associate professor Xuefei Xu’s group at the Center for Combustion Energy and Department of Energy and Power Engineering of Tsinghua University. The title of this paper is ‘Mechanistic insights into trisulfur radical generation in lithium-sulfur batteries’.
As next-generation battery systems, lithium-sulfur batteries possess an ultra-high theoretical energy density (2600 Wh/kg). However, the slow sulfur-conversion reaction rate on the cathode side of lithium-sulfur batteries affects the cycle performance and impedes the commercialization of batteries. As crucial intermediates in sulfur-conversion reactions, trisulfur radicals can activate new reaction pathways to facilitate the conversion of sulfur species in liquid electrolytes. Therefore, understanding the reaction mechanism related to trisulfur radicals is of great significance for improving the kinetics of sulfur-conversion reactions. Existing studies have proved that trisulfur radicals are generated through the bond cleavage of hexasulfides, but whether this generation reaction can occur in liquid electrolytes is highly dependent on the properties of solvents. For example, in dimethyl sulfoxide (DMSO) solvents with a high dielectric constant/donor number, trisulfur radicals can be clearly detected. While in 1,3-dioxolane (DOL) solvents with a low dielectric constant/donor number, these radicals are almost absent. In order to explain this solvent-dependent phenomenon, this work conducted a systematic theoretical exploration from both thermodynamic and kinetic perspectives based on density functional theory.
As shown in Figure 1, two types of reactions are identified to be involved in the conversion from hexasulfides (Li2S6, LiS6-, and S62-) to trisulfur radicals (LiS3 and S3-). The first type (R1-R3) refers to the delithiation of hexasulfides, and the second type (R4-R9) corresponds to the decomposition of hexasulfides to form trisulfur radicals. By studying the solvation behavior of all relevant species (Li2S6, LiS6-, S62-, LiS3, S3-, and Li+), the lithium-solvent interactions are found to play a key role in the solvation of lithium-containing species (Li2S6, LiS6-, LiS3, and Li+). The lithium-centered tetrahedral coordination solvation structures exhibit the highest stability. For S62- and S3- without lithium, their solvation is energetically unfavorable.
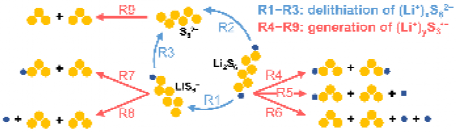
Figure 1. Relevant reactions in the conversion from hexasulfides to trisulfur radicals
Next, the calculated reaction free energies of the first type of reactions indicate that the preferential lithiation states of hexasulfides in DMSO and DOL are different. DMSO solvents with a higher dielectric constant and donor number and accordingly with stronger lithiophilicity contribute to the greater polarity and weakness of Li-S bonds, so that Li2S6 delithiate to form LiS6- and S62- more easily. On the contrary, in DOL, Li-S bond breaking is rather improbable and Li2S6 thereby remains neutral. Whether in DMSO or in DOL, with the increased number of coordinated solvents of hexasulfides, the reaction free energies thereby decrease.
The authors then conducted a thermodynamic analysis of the second type of reactions in DMSO and DOL solvents. Results demonstrate that trisulfur radicals are very likely to be produced in DMSO, and the generation would be thermodynamically easier when hexasulfides are more fully solvated. However, the formation of trisulfur radicals is always unfavorable in DOL. Combined with a kinetic analysis, possible generation mechanisms of trisulfur radicals from hexasulfides were proposed. As depicted in Figure 2, the whole reaction pathway in DMSO involves steps such as (de)solvation, delithiation, Li-S bond cleavage, and S-S bond cleavage. The maximum free energy barriers along the proposed pathways are computed to be 9.62 kcal/mol in DMSO and 19.08 kcal/mol in DOL, respectively.
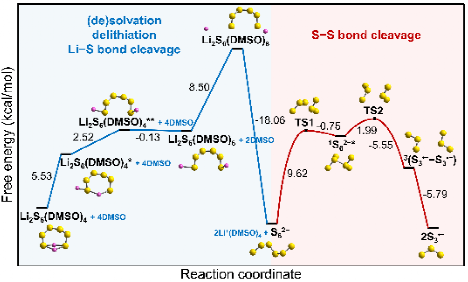
Figure 2. Proposed generation mechanism of trisulfur radicals in DMSO
To validate the generalizability of the findings in this work, the authors chose DMA, another high dielectric constant/donor number solvent, and got very similar results to those in DMSO. The authors also explored the generation mechanism of tetrasulfur and disulfur radicals from hexasulfides, and confirmed that the formation of these two kinds of radicals is unfavorable in both DMSO and DOL.
In summary, the authors analyzed the solvation behavior of sulfur species in liquid electrolytes from a theoretical perspective, revealed the generation mechanism of trisulfur radicals, and associated the mechanism with the properties of solvents. This work is expected to enlighten the electrolyte design toward fast kinetic sulfur-conversion in Li-S batteries.
Xu Han, a PhD student in the Class of 2018, is the first author of this paper, and associate professor Xuefei Xu is the corresponding author. This work was supported by the National Natural Science Foundation of China (21973053) and the Creative Seed Fund of Shanxi Research Institute for Clean Energy, Tsinghua University.
The link for this paper: https://doi.org/10.1039/D3TA03366J
Provided by Xuefei Xu Research Group
Approved by Yu Cheng Liu, Xiaoqing You


