New Advances in the Study of Flame-Synthesized Copper-Ceria Nanomaterials for Mercury Removal
by the Research Group of Prof. Xiaoqing You from the Center for Combustion Energy at Tsinghua University
Recently, a series of collaborative research work by Associate Professor Xiaoqing You from the Center for Combustion Energy and Professor Shuxiao Wang from the School of Environment at Tsinghua University has been published in the journals "Chemical Engineering Journal" and "Fuel". The titles of the papers are "Flame-made high-capacity and highly efficient nanomaterial CuOx-CeOx-WO3/TiO2 for mercury adsorption" and "Copper-ceria catalysts with improved water and sulfur resistance: Computational design and structural optimization".
Copper-ceria catalysts, which contain Ce4+/Ce3+ redox couple, exhibit excellent catalytic activity in the catalytic oxidation and removal of elemental mercury. The introduction of copper oxide to form copper-ceria binary catalysts improves their sulfur resistance, but the active sites of the catalyst tend to collapse at high temperatures. In order to prepare water-thermally stable copper-ceria-based catalysts, the authors synthesized copper-ceria nanomaterials in a high-temperature flame. As shown in Figure 1, the copper-ceria catalyst synthesized by laminar premixed flames (Cu10Ce10W9Ti-F) demonstrated better mercury oxidation performance and stronger water-thermal stability compared to the catalyst synthesized by the impregnation method (Cu5Ce5W9Ti-I). This can be attributed to the more stable structure and the formation of more metal-support interactions in the former.
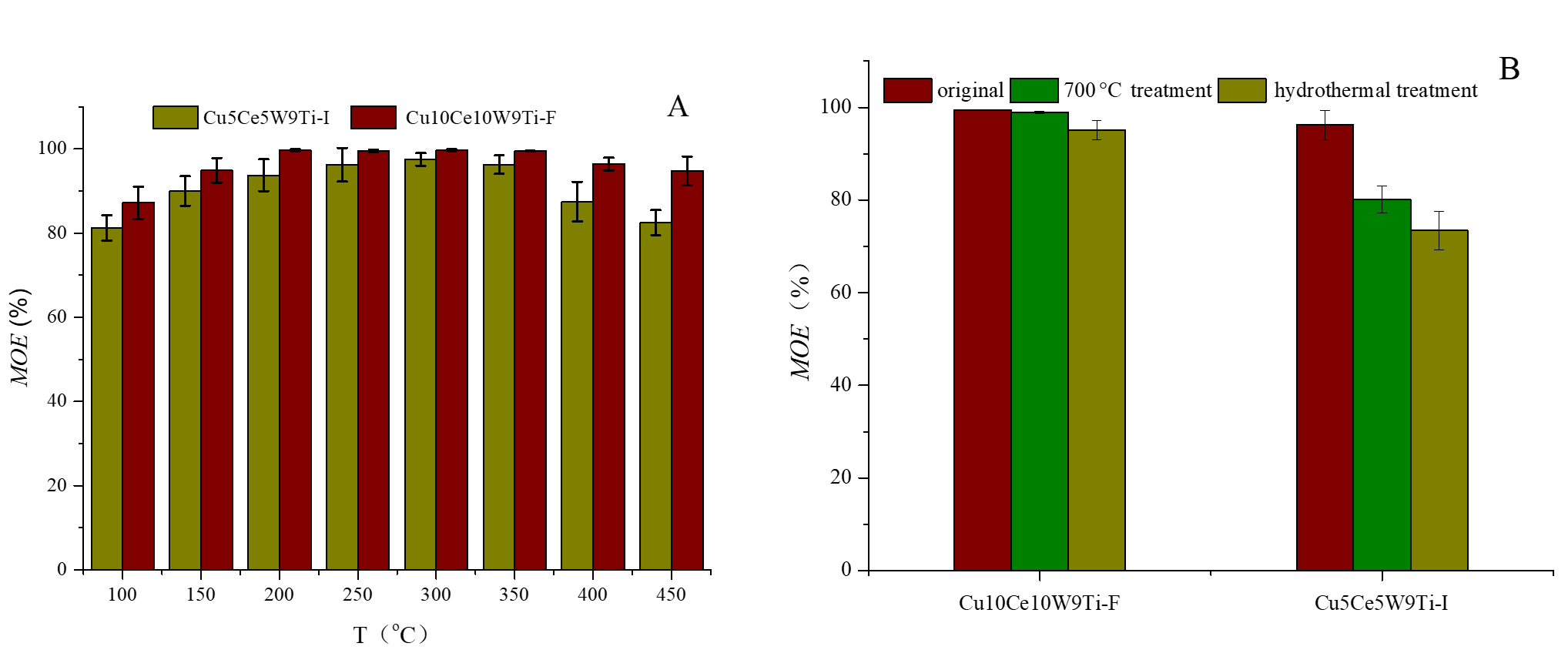
Figure 1. Comparison of Mercury Removal Performance between Copper-Ceria-Based Catalysts Synthesized by Laminar Premixed Flames (Cu10Ce10W9Ti-F) and the Impregnation Method (Cu5Ce5W9Ti-I)
To further enhance copper dispersion, the authors utilized a flame spray pyrolysis (FSP) burner with a higher flame temperature to prepare a highly efficient and high-capacity mercury adsorbent (CuCeWTi-FSP). As shown in Figure 2, the efficiency of mercury adsorption remained above 90% even after 30 days. Under conditions with the presence of oxygen at temperatures ranging from 100 to 800°C, the efficiency of Hg0 adsorption was consistently 100%. At 450°C, the mercury adsorption capacity reached 49,000 μg/g while maintaining a high efficiency of 95%, significantly outperforming the adsorbent prepared by the impregnation method.
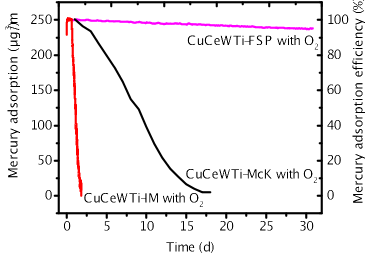
Figure 2. Comparison of mercury adsorption efficiency for CuCeWTi-IM, CuCeWTi-McK and CuCeWTi-FSP (Basic reaction condition: N2 + 6% O2 ; Hg0 concentration: 250.0 μg/m3; space velocity: 100,000 h-1 )
The authors also conducted an analysis of the surface elemental composition and valence state changes before and after mercury adsorption on the adsorbent. Combined with computational findings, it was discovered that on the surface of CuCeWTi-FSP, molecular oxygen first adsorbs onto copper atoms, then decomposes into adsorbed oxygen atoms, followed by the adsorption and oxidation of mercury atoms. On the other hand, on the surface of CuCeWTi-IM, mercury adsorption depletes the surface active oxygen, leading to a rapid saturation of the adsorbent, as shown in Figure 3. Therefore, CuCeWTi-FSP exhibits a stronger mercury adsorption capacity.
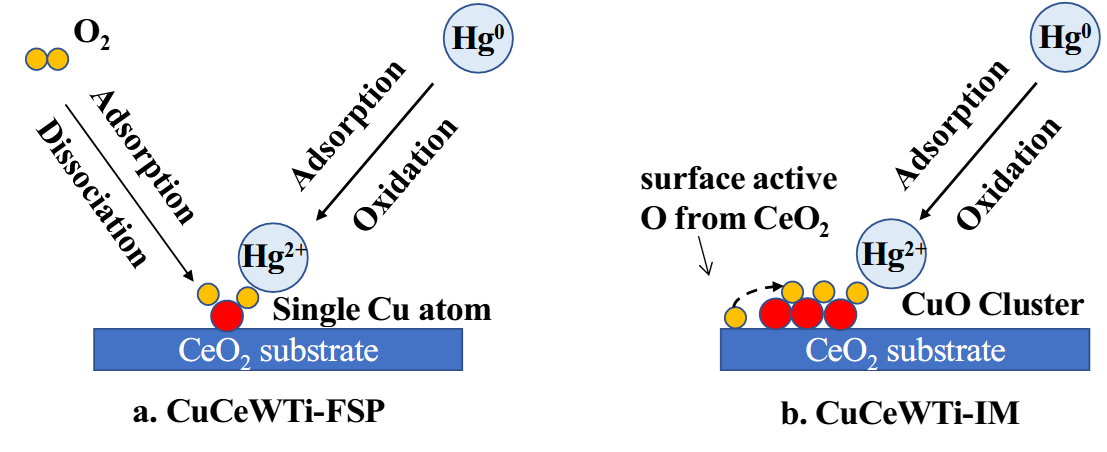
Figure 3. Mercury adsorption processes on CuCeWTi-IM and CuCeWTi-FSP surfaces.
To analyze the differences in mercury oxidation and water resistance between the two catalysts, the authors employed density functional theory calculations to study the mercury oxidation process and the influence of SO2 and H2O on the surfaces of the catalysts. Figure 4 shows the mercury adsorption energy at different sites on cluster copper and highly dispersed copper surfaces. The results indicate that highly dispersed copper can adsorb more mercury by utilizing molecular oxygen in the air. Furthermore, in the presence of surface water, the hydrolysis process consumes oxygen atoms from the adsorbed molecular oxygen, while cluster copper requires the consumption of active surface oxygen. Therefore, highly dispersed copper exhibits stronger water resistance.
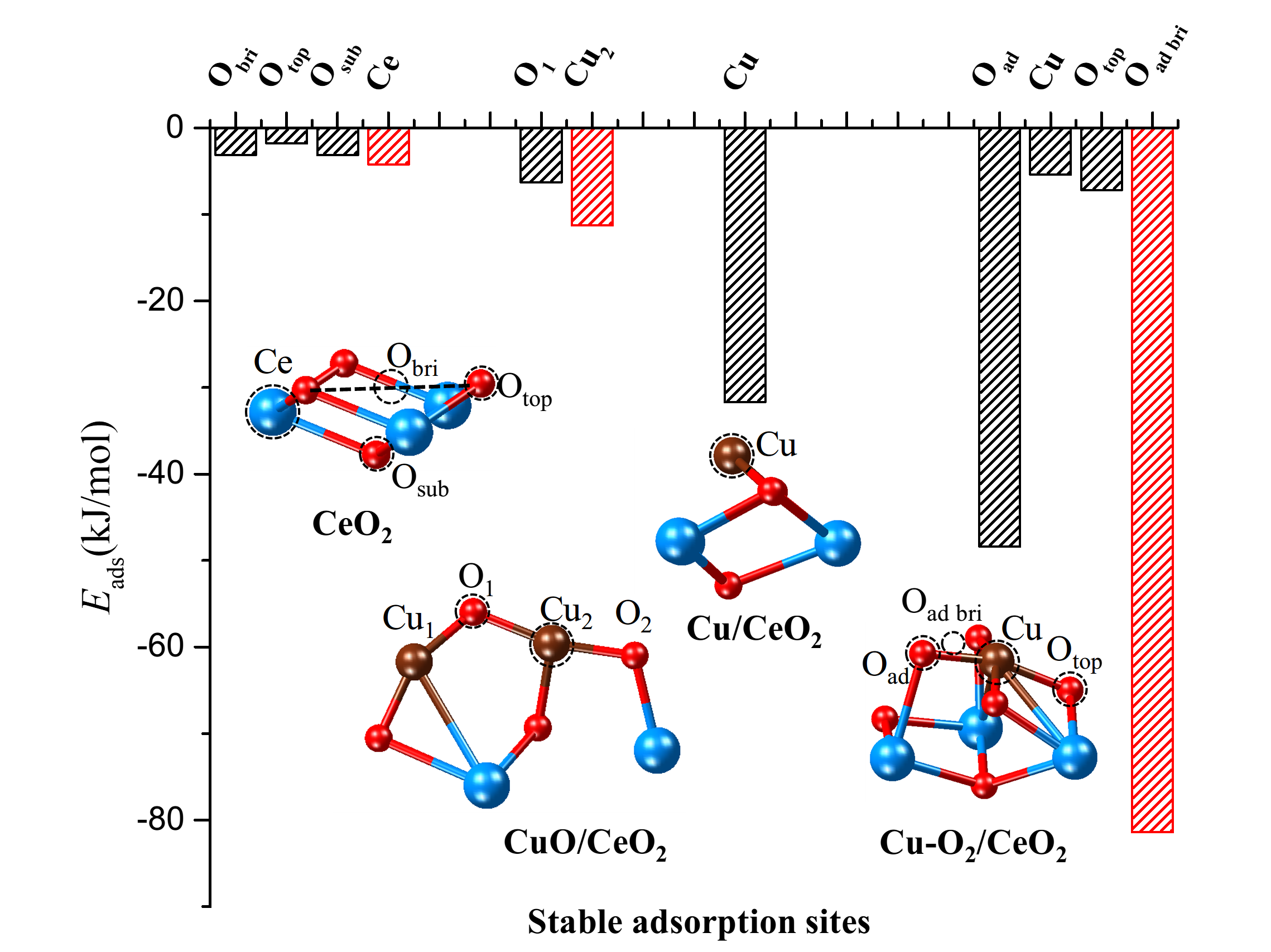
Figure 4. The adsorption sites and adsorption energies of Hg0 on the surfaces of CeO2, Cu/CeO2, CuO/CeO2, Cu-O2/CeO2 (The results of the most stable adsorption sites are marked in red.)
Summary: In this study, a flame-synthesized catalyst with highly dispersed copper on ceria surface was obtained, which achieved efficient and high-capacity mercury adsorption over a wide temperature range. The mercury adsorption capacity and the mechanism of water and sulfur resistance of different copper-ceria binary structures were theoretically investigated.
The first author of the two papers is Sen Shao, a Ph.D. student from the Center for Combustion Energy at Tsinghua University. Associate Professor Xiaoqing You is the corresponding author of the papers, and Professor Shuxiao Wang from the School of Environment is the co-corresponding author of the first paper. The article links are as follows.
https://doi.org/10.1016/j.cej.2024.148835
https://doi.org/10.1016/j.fuel.2023.129571
Provided by Xiaoqing You Research Group
Approved by Yu Cheng Liu, Xiaoqing You


