A paper titled Catalytic combustion of NH₃ in Pt-coated microchannels: A numerical study on the surface-gas chemistry coupling and its impact on product selectivity is recently published by the CCE Sui Group in the journal Combustion and Flame. This work presents a detailed investigation of the roles of gaseous chemistry and surface-gas coupling on the efficiency and product selectivity of ammonia (NH₃) oxidation in catalytic microchannels. The study reveals that through catalytic-gaseous chemistry coupling, it is possible to achieve both low-temperature stable combustion and low NOx emissions. These findings may provide guidance for the design and optimization of industrial ammonia combustors.
Increasing global demands for zero-carbon energy have brought ammonia, as a fuel or fuel additive in future energy systems, into the spotlight. Ammonia has high octane numbers and low flame temperatures, making it suitable for high compression ratio combustion in internal combustion engines. Compared to hydrogen, ammonia also offers advantages in storage and transport. However, direct utilization of ammonia combustion still faces several challenges, such as difficulty in ignition, a narrow flammability range, and most importantly, the issue of NOx emissions. Catalytic combustion presents an effective solution to these problems. With its inherently low activation energy, high combustion stability, and minimal NOx emissions when burning hydrogen and hydrocarbon fuels, catalytic combustion has been widely applied in large- and small-scale power generation, fuel processing, emission control, and chemical synthesis.
Research on catalytic combustion of ammonia is still in its early stages. Existing studies have primarily focused on selective catalytic oxidation (SCO) of NH₃ or the Ostwald process for NO production, rather than on the use of ammonia as a fuel for energy supply through catalytic combustion. In response, this study performs numerical simulations of lean premixed NH₃/O₂/N₂ combustion in a platinum (Pt)-coated catalytic microchannel, targeting applications such as small- to medium-scale power generation using ammonia as a fuel.
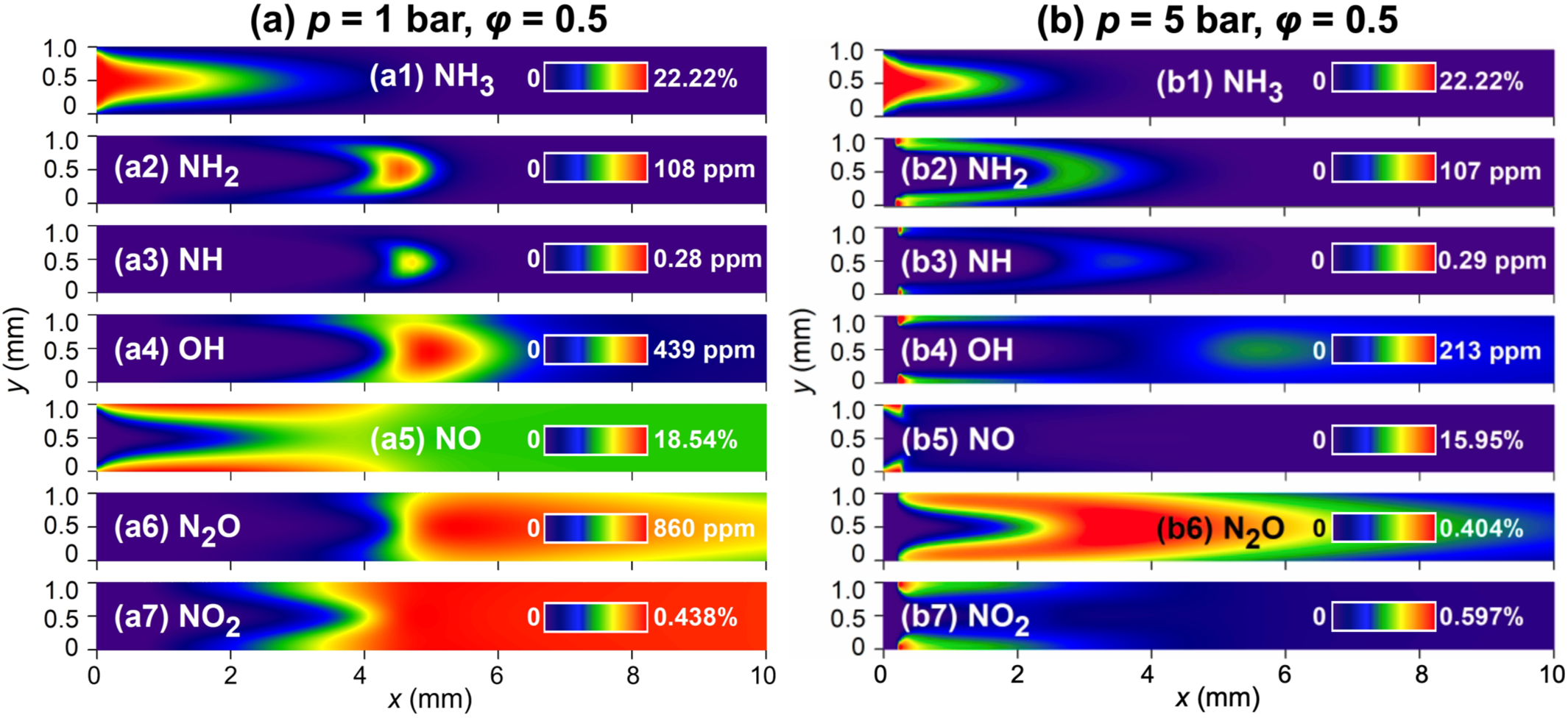
Figure 1: 2D distributions of major gas-phase species in the Pt channel with wall temperature of 1300 K.
Through sensitivity analysis, surface coverage, and reaction pathway analysis, the study reveals the mechanisms behind the formation of large amounts of NO via surface reactions and how gas-phase reactions facilitate the conversion of NO to N₂. Due to the high coverage of surface oxygen O(s), ammonia undergoes successive dehydrogenation on the catalyst surface, reacts with O(s), leading to significant NO formation. Meanwhile, NH₂ radicals in the gas phase consume NO through reactions NH₂ + NO → N₂ + H₂O and NH₂ + NO → NNH + OH. These reactions, followed by a series of elementary steps, promote the further conversion of radicals to molecular nitrogen.
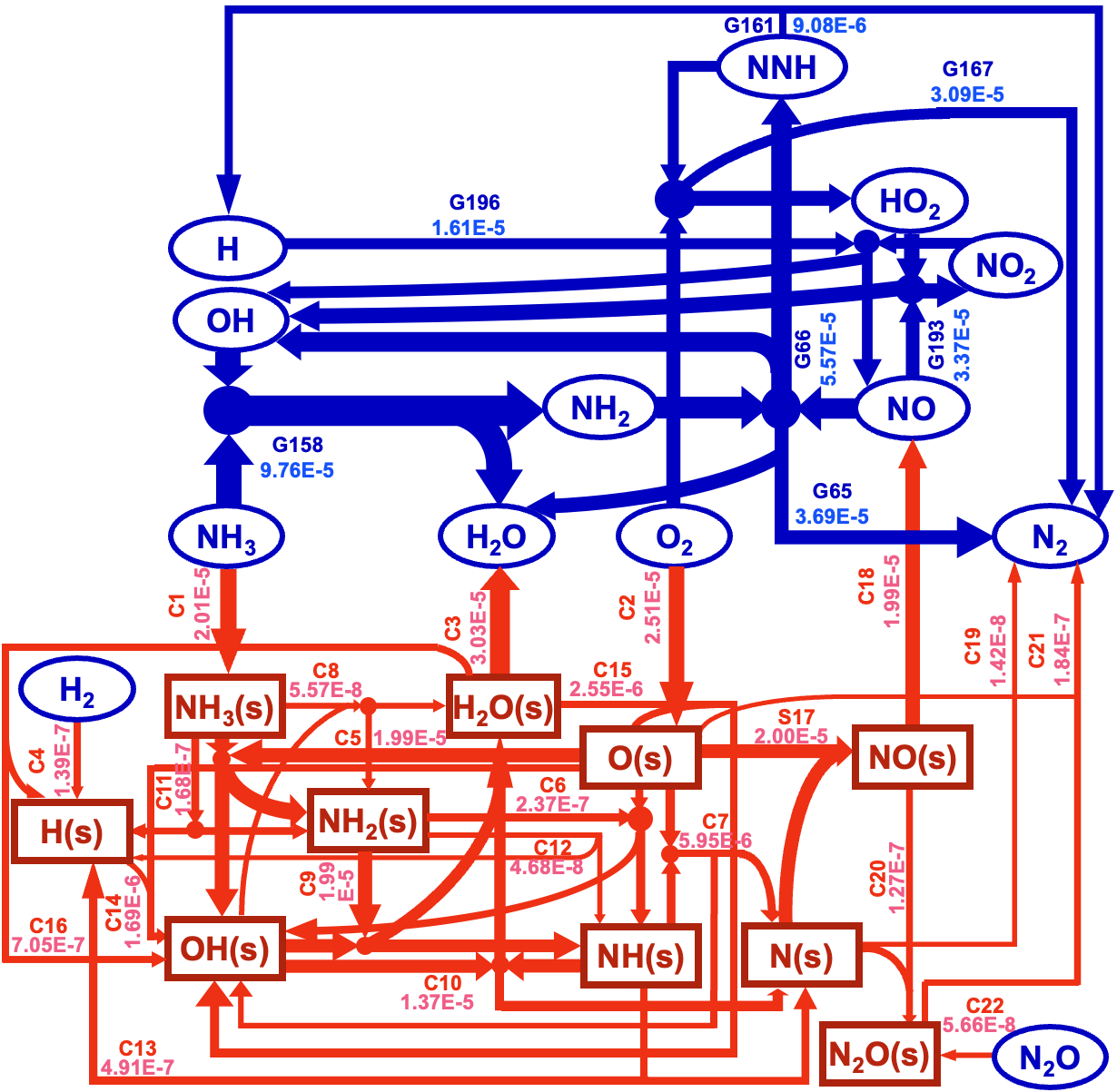
Figure 2: Reaction Pathway Diagram at Tw = 1300 K, p = 1 bar, and φ = 0.5
This research was supported by the National Natural Science Foundation of China (NSFC) and Dushi Project of Tsinghua University.
Provided by: The CCE Sui Group
Approved by: Yu Cheng Liu, Xiaoqing You


