Recently, a paper was published in Journal of Colloid and Interface Science by Yawen Gao and others from Professor Chao Sun's group at the Center for Combustion Energy and Department of Energy and Power Engineering of Tsinghua University. The paper, titled "Ionic environment-modulated nucleation and stability of multiscale nanodomains in surfactant-free microemulsions", details their latest findings.
Nanodroplets are important dispersed systems with wide-ranging industrial applications, including petroleum extraction, drug delivery, and cosmetics manufacturing. The aqueous environment significantly influences the nucleation and growth of nanodroplets. A deep understanding of these dynamic mechanisms is crucial for the controlled preparation of nanodroplets. Traditional microemulsions stabilize oil and water components through adding the surfactants, but surfactant-free microemulsions offer a more environmentally friendly and potentially safer alternative. Professor Sun’s group innovatively constructed a ternary surfactant-free microemulsion system composed of trans-anethole (oil phase), ethanol and water. They employed multi-scale characterization approaches, including a self-built nanoparticle tracking analysis (NTA, Figure 1), dynamic light scattering (DLS) and dielectric constant measurements, supported by theoretical calculations. This comprehensive approach systematically revealed the mechanism by which the ionic environment (including pH and electrolyte) modulates the stability of nanodroplets.

Figure 1. Self-built nanoparticle tracking analysis (NTA) system.
The spontaneous formation of multi-scale nanodroplets was successfully observed in the pre-Ouzo single-phase region of ternary phase diagram. The study reported that in oil-in-water (O/W) systems, nanodroplet sizes ranged from molecular-level nanoclusters (approximately 1 nanometer) to mesoscopic droplets (approximately 200 nanometers). In contrast, water-in-oil (W/O) systems primarily showed uniform nanostructures/aggregates (approximately 3 nanometers), exhibiting unique high stability against pH regulation.
The research indicates that near-neutral conditions are most favorable for nanodroplet nucleation and stability (with the highest absolute value of zeta potential). Extreme acidic or alkaline conditions significantly reduce the surface charge of nanodroplets (zeta potential approaches zero), weakening electrostatic repulsion between droplets and thus enhancing the instability of the microemulsion system. As acidity or alkalinity increases, mesoscopic droplets grow larger and their number concentration decreases (Figure 2A), promoting droplet coalescence and phase separation. Beyond pH control, the study also found that adding strong electrolytes like sodium chloride leads to a "salting out effect" and weakens electrostatic repulsion, resulting in large droplets with low number density, further promoting oil-water phase separation.
The research group experimentally confirmed that the time evolution of droplet growth primarily follows the Ostwald ripening mechanism. Experimental measurements aligned with theoretical calculations within the set tracking time, showing that high acidity or alkalinity led to faster droplet growth rates (Figure 2B). Under neutral conditions, the electrostatic repulsion generated by the accumulation of surface charges effectively counteracts Laplace pressure, reducing the growth rate and leading to slow droplet growth.
This work not only deepens the theoretical understanding of nanodroplet stability modulation mechanisms but also provides crucial theoretical guidance and technical support for the controlled preparation of nanodroplets in related fields such as drug solubilization, cosmetics manufacturing, food emulsification, and environmental remediation.
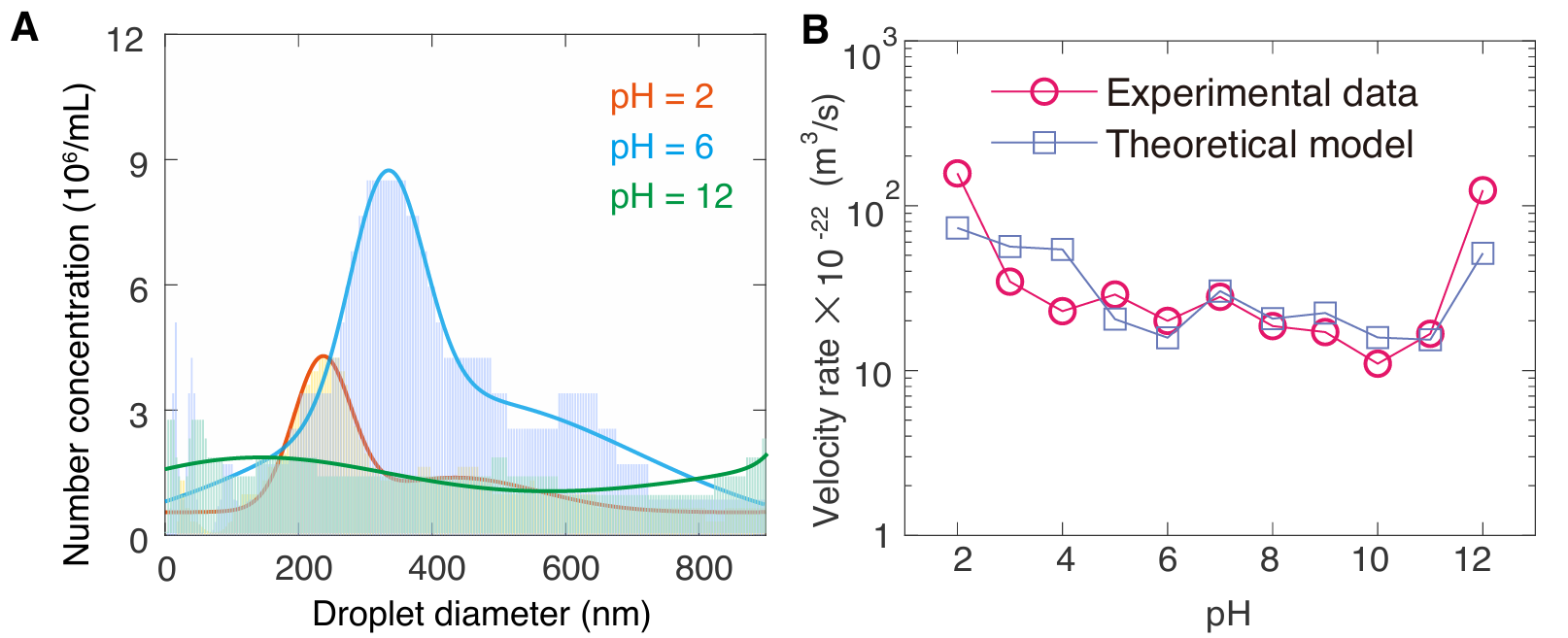
Figure 2. (A) Effect of acid-base regulation on nanodroplet number concentration; (B) Experimental measurements and theoretical calculations showing the trend of nanodroplet growth rate as a function of aqueous conditions.
The first author of this paper is Dr. Yawen Gao, a Postdoc Fellow at Tsinghua University. Professor Chao Sun and Dr. Mingbo Li, Assistant Researcher at Shanghai Jiao Tong University, are corresponding authors. Collaborators also include Dr. Changsheng Chen, a Postdoc Fellow at Tsinghua University. This research was supported by the National Natural Science Foundation of China and the SINOPEC Petroleum Exploration and Production Research Institute.
Article Link:https://www.sciencedirect.com/science/article/pii/S002197972501224X
Provided by: Professor Chao Sun's Research Group
Approved by: Yu Cheng Liu, Xiaoqing You


