Recently, Researcher Dong Su from the Institute of Physics, Chinese Academy of Sciences, Associate Professor Liang Zhang from the Center for Combustion Energy / School of Vehicle and Mobility, Tsinghua University, and Researcher Dan Zhou from the Leibniz Institute for Crystal Growth have made new progress in the study of metal–support interactions in heterogeneous catalysts during redox reactions. The related work, entitled “Looping metal–support interaction in heterogeneous catalysts during redox reactions”, has been published online in Nature Communications.
Heterogeneous catalysts play a crucial role in a wide range of chemical processes, including chemical synthesis, energy conversion, and environmental remediation. In metal–oxide support systems, the oxide serves multiple key functions: it stabilizes metal nanoparticles under extreme conditions and modulates catalytic behavior through metal–support interactions (MSIs). These interactions are primarily governed by metal–metal or metal–oxygen interactions at the interface, giving rise to complex phenomena such as electronic metal–support interactions (EMSI), strong metal–support interactions (SMSI), and reactive metal–support interactions (RMSI). Such MSIs profoundly influence catalytic performance, directly determining critical parameters—such as activity, selectivity, and stability—in diverse reactions including hydrocarbon dehydrogenation, Fischer–Tropsch synthesis, catalytic oxidation, gas- and liquid-phase reforming, and selective hydrogenation. Therefore, understanding the interfacial structure and achieving precise control over MSIs has become a pivotal strategy for optimizing catalytic performance.
This study employed environmental transmission electron microscopy (ETEM) to investigate the reaction kinetics of the NiFe–Fe₃O₄ catalyst during the hydrogen oxidation reaction (H₂ + O₂ → H₂O) and revealed a novel looping metal–support interaction (LMSI). By combining DFT+U calculations with neural network molecular dynamics (NN-MD) simulations, the work systematically elucidates the spatially decoupled hydrogen oxidation mechanism driven by LMSI in the NiFe–Fe₃O₄ system. The simulations show that the NiFe–Fe₃O₄ system exhibits an exceptionally low H₂ dissociation barrier (0.11 eV), significantly lower than those of Fe₃O₄ and Fe–Fe₃O₄ systems. The activated hydrogen atoms spill over along the interface, promoting the reduction of Fe₃O₄ and triggering an oxygen reverse spillover effect, whereby oxygen preferentially migrates to the NiFe surface and reacts with dissociated hydrogen to form water. Meanwhile, the reduced Fe atoms rapidly migrate along the Fe₃O₄ {111} facets and are re-oxidized in regions distant from NiFe, achieving a spatially separated redox cycle. The superior H₂ dissociation activity of NiFe, together with the fast surface diffusion of Fe atoms, establishes a broad hydrogen-rich region and an extended Fe migration pathway, enabling efficient spatially decoupled redox kinetics. This work highlights the critical role of LMSI in regulating the hydrogen oxidation reaction pathway and provides new theoretical guidance and design strategies for metal–oxide interfaces in heterogeneous catalysis.
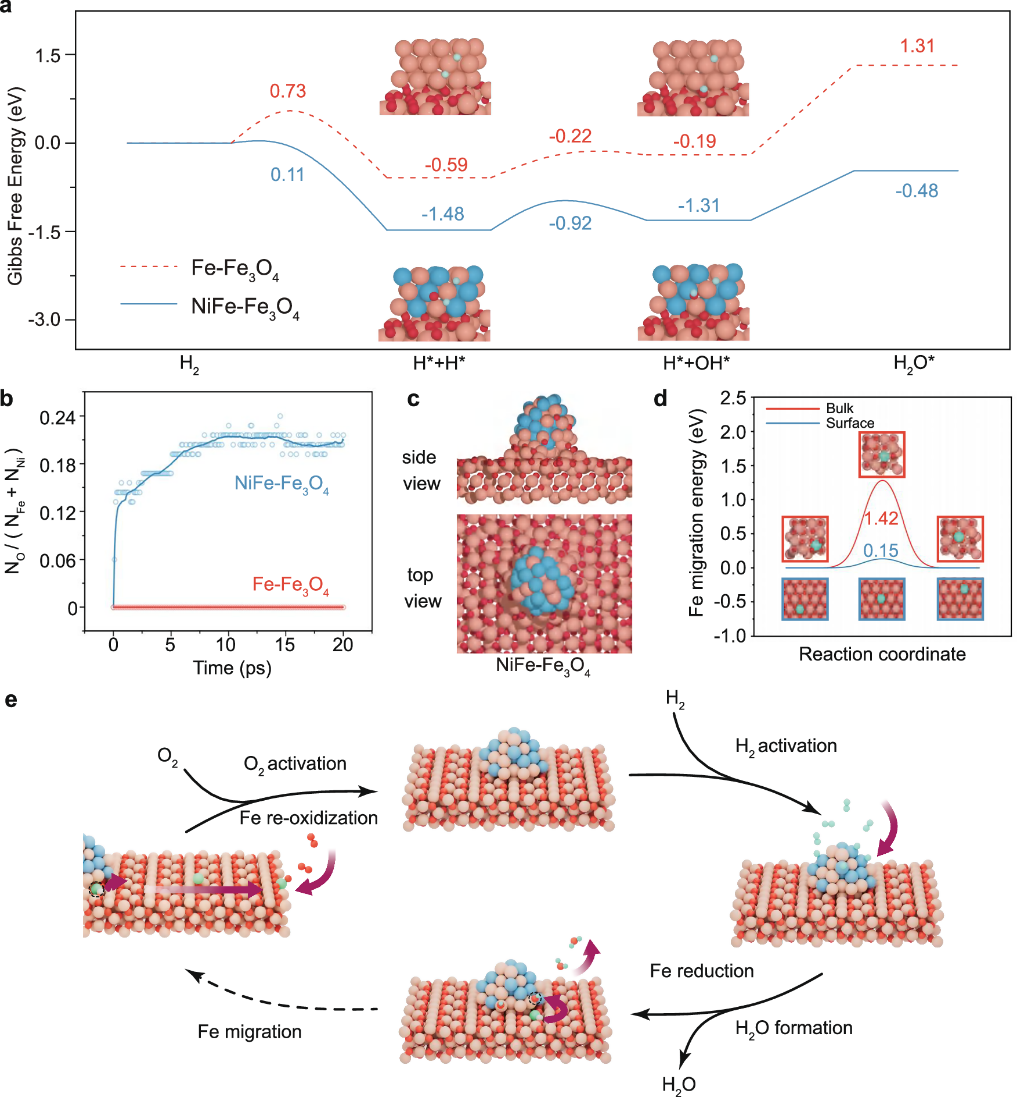
Figure: Theoretical analysis of the atomic-scale reaction pathway illustrating the LMSI mechanism on the NiFe–Fe₃O₄ catalyst.
Ph.D. student Yue Pan from the Institute of Physics, Chinese Academy of Sciences, and 2022 Ph.D. student Shiyu Zhen from the Center for Combustion Energy / School of Vehicle and Mobility, Tsinghua University, contributed equally as co-first authors of this paper. Researcher Dong Su from the Institute of Physics, Chinese Academy of Sciences, Associate Professor Liang Zhang from the Center for Combustion Energy / School of Vehicle and Mobility, Tsinghua University, and Researcher Dan Zhou from the Leibniz Institute for Crystal Growth, Germany, served as co-corresponding authors. This work was supported by the National Natural Science Foundation of China and other funding programs.
Provided by: Liang Zhang's Group
Approved by: Yu Cheng Liu, Xiaoqing You


